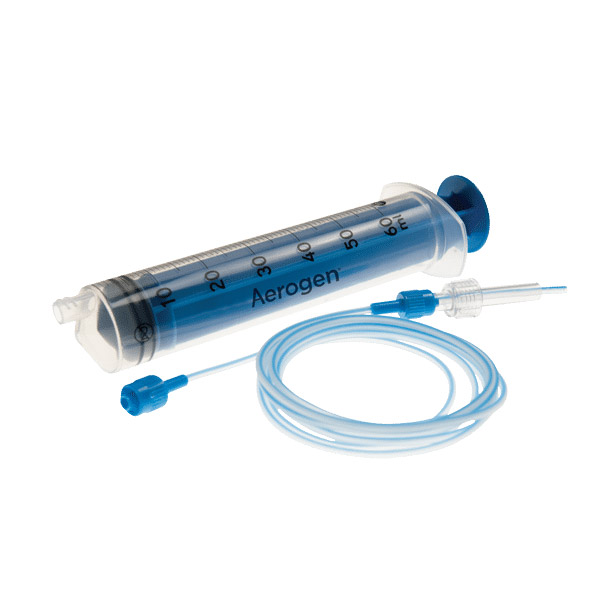
Set di deflussori Aerogen per nebulizzazione continua, per un controllo preciso del farmaco goccia a goccia per la nebulizzazione continua2
- Versatile e compatibile
Funzionamento con la maggior parte delle pompe a siringa standard2 - Funzionalità di sicurezza integrate
Adattabile alle pompe a siringa standard, il CNTS è dotato di connettori non standard specializzati per contribuire a ridurre il rischio di collegamenti errati, con deflussori blu unici e dotati di codice colore per una facile identificazione 1
Come funziona
Un sistema monopaziente che consente la nebulizzazione continua e un controllo preciso goccia a goccia della somministrazione dei farmaci ai pazienti.2
Aerogen CNTS
Supporto relativo al prodotto
Aerogen Set Up - Aerogen Ultra with a Mask
Aerogen Set Up - Aerogen Ultra with a Mouthpiece
Aerogen Set Up - NIV
Aerogen Set Up - Dry Side of the Humidifier
Aerogen Set Up - Adult Nasal High Flow
Aerogen Set Up - Paediatric Nasal High Flow
Aerogen Set Up - At The Wye
Aerogen Set Up - CNTS
General
I have a medical/clinical query, or my query is not addressed below. Who can I contact for medical or clinical information related to the use of Aerogen products?
Please contact Aerogen Medical Affairs directly for medical information/published clinical research regarding the use of Aerogen products. Email: MedicalScience@aerogen.com (US/Canada/LATAM) or msl@aerogen.com (all other countries).
What medications can be used with the Aerogen Solo, Aerogen Pro?
The Aerogen Solo & Aerogen Pro should be used with physician prescribed medications for inhalation which are approved for use with a general purpose nebuliser.¹ Should you require information on a whether a medication is approved for inhalation please contact the medication manufacturer directly.
Should I adjust the drug dosage when I’m using the Aerogen Solo, Aerogen Pro?
Information on drug dosing must be sourced from the manufacturer’s approved prescribing information for the inhaled formulation. Aerogen Ltd cannot provide specific advice on medication dose.
Is the drug affected if it causes discoloration of the water in a humidifier?
Occasionally, a change in the colour of the water in a heated humidifier has been noted with certain medications if aerosolized proximal to the humidifier. Should you have concerns related to a specific medication please do not hesitate to contact the Medical Affairs team at the email addresses listed above for assistance. The treating clinician should always consider a medications SPC and/or consult with a pharmacist/hospital pharmacy to determine whether placement proximal to the humidifier is suitable for a given therapeutic. Aerogen devices are approved for use in a number of positions offering the treating health care professional a range of placement options if discoloration is a concern. These positions are detailed in the directions for use documents which are accessible on the website.¹
What do I do if crystals form in the nebuliser chamber?
Any medication that has a high ionic concentration, for instance hypertonic saline solutions, if permitted to dry on the Aerogen Vibronic aerosol generator in the nebuliser chamber, may form visible crystals. In the event where crystallisation is observed in the Aerogen Solo, Aerogen recommend to aerosolise a few drops of normal saline solution to clear any residual crystallisation.
How do you remove residue in the chamber after nebulisation of viscous drugs?
Should you wish to remove any viscous drug residues from the Aerogen Solo medication reservoir you may nebulise a few drops of normal saline.
What effect does bias ventilator flow have on aerosol output and deposition?
The impact of bias ventilator flow on aerosol deposition depends on the location of the aerosol generator in the circuit. An in vitro study by Ari et al., (2010) assessing aerosol delivery during mechanical ventilation in the presence of bias flow determined that optimal aerosol deposition may be achieved by nebuliser placement on the dry side of humidifier. Similarly, Berlinski & Willis (2013) demonstrated in a paediatric model, that in the presence of bias flow, nebulisers were more effective when placed back at the humidifier as compared to closer to the wye.²⁻³ In the absence of bias flow optimal deposition was observed when the nebuliser was placed at or close to the wye in this adult model.⁴
Can you use a Heat and Moisture Exchange Device (with filter) (HME/HMEF) while delivering aerosol with the Aerogen Solo?
Yes, the Aerogen Solo is approved for use with a HME.¹ The Aerogen Solo should be placed between the patient and HME as detailed in the Aerogen Solo directions for use.¹ Note: Only a HME/HMEf approved for use with a nebuliser should be used. Follow the HME/HMEf manufacturer instructions regarding use with a nebuliser. Ensure the combination of nebuliser, T-piece and HME/HMEf volumes are suitable for the tidal volume being delivered, according to the patient size.
What are the advantages of Aerogen vibrating mesh nebulisers (VMN) compared with other nebulisers, or inhalers for adult and pediatric patients?
Aerogen vibrating mesh nebulisers are electrically powered, quiet and require no additional flow or pressure to operate, ensure a minimal residual volume and are suitable for use with physician prescribed solutions that are approved for use with a general-purpose nebulisers.¹ A narrative review by Lin et al. compared VMN with other, similar, during invasive mechanical ventilation.⁵
Can you deliver an effective dose with the Aerogen Solo to an adult patient during High flow therapy?
Both in vitro and in vivo studies have examined aerosol delivery efficiency via Aerogen devices concurrent with High Flow Nasal Oxygen therapy with adult models/patients⁶⁻¹⁰ These studies suggest that an effective dose may be delivered during concurrent High Flow Nasal Oxygen therapy and medication aerosolisation via the Aerogen Solo.⁶⁻¹⁰
Do different ventilator parameters affect aerosol delivery?
Yes, a range of ventilator parameters; including flow, inspiratory time (duty cycle), ventilatory mode, timing of nebulisation and circuit features; tube sizes, heat, and humidification, can affect aerosol delivery in mechanically ventilated patients.2,11-13 Reviews by Dr. Dhanani and others summarise the impact of these features on aerosol delivery.11,13
Aerogen Continuous Nebulisation Tube Set (CNTS)
I have a medical/clinical query, or my query is not addressed below. Who can I contact for medical or clinical information related to the use of Aerogen products?
Please contact Aerogen Medical Affairs directly for medical information/published clinical research regarding the use of Aerogen products please email: MedicalScience@aerogen.com (US/Canada/LATAM) or msl@aerogen.com (all other countries).
How do I setup the Aerogen CNTS with an infusion pump?
Insert the syringe filled with medication into the syringe infusion pump and set the appropriate flow rate (refer to pump manual or manufacturer for guidance). To ensure correct and safe connection between the nebuliser and the medication reservoir, trace the medication tube from the nebuliser back to the medication reservoir to make sure the medication tube is connected to the correct source.¹
What syringe pump software should you use with the Aerogen CNTS?
The recommended syringe pump software setting with the Aerogen syringe is typically the “BD” or the “BD Plastipak” setting. This must be validated locally before use. Refer to pump manual or manufacturer for guidance. These pumps should also be used in accordance with local hospital or ward policies.¹
What flow rate should I use with the Aerogen CNTS?
The flow rate should be selected based on the clinical need of the patient and the clinician’s judgement. The maximum input rate of medication into the Aerogen Solo nebuliser during continuous nebulisation is up to 12 mL per hour.1 The upper limit of 12 mL per hour is based on Aerogen’s specification for the minimum nebuliser flow rate and should not be exceeded.
Is there a minimal flow rate or how low can I run the Aerogen CNTS?
There is no minimum flow rate. Aerosol will be produced as it comes into contact with the aperture. At low flow rates a pause between visible aerosol generation will be apparent as each drop is nebulised as it contacts the Aerogen Vibronic aperture plate.
How does volumetric dosing work with Aerogen CNTS?
The CNTS delivers medication on a drop-by-drop basis onto the vibrating mesh. The rate of drug entering the nebuliser determines the rate of aerosolization or drug output rate. This gives you the ability to titrate medication utilizing the infusion rate of the pump. If the infusion rate is low, you may see pauses between visible aerosol being generated, however this is a normal observation at low flow rates and there is no reason for concern.
Are pauses in visible aerosol generation normal with volumetric dosing?
Yes. Medication is delivered drop by drop with volumetric dosing. When solution drops on to the aperture plate aerosol is produced immediately. A pause in visible aerosol generation is to be expected with drop-by-drop volumetric delivery.
Can you provide an example of a medication calculation for volumetric dosing?
Aerogen Medical Affairs can assist you directly should you require information on how to undertake a calculation for volumetric dosing. Please contact them directly at: MedicalScience@aerogen.com (US/Canada/LATAM) or msl@aerogen.com (all other countries).
Why is the fluid level in the aerosol chamber rising?
Rising fluid level in the aerosol chamber may indicate that the input fill rate has exceeded the nebulisation rate of the Solo. The recommended input rate of medication into the Aerogen Solo nebuliser during continuous nebulisation is up to a maximum of 12 mL per hour and should not be exceeded.¹ Rising level of medication in the Aerogen Solo medication reservoir may also occur if the Aerogen Solo nebuliser is turned off while the feed system is still on, or the nebuliser is not in its recommended orientation.¹
How do I fill the Aerogen Syringe?
Use another syringe to draw up the medication with a needle or needleless connector and with the plunger pulled back on the Aerogen syringe insert the medication through the cap end of the Aerogen syringe.
Can I reuse the Aerogen Syringe?
The Aerogen syringe is intended as a single-use item and should be disposed of after use.
What is the priming volume of the Aerogen Continuous Nebulisation Tube Set tubing?
You should prime the tubing until the medication reaches the end of the tubing. The maximum tubing priming volume is maximum 3.65 mL.¹ Please ensure that all air is removed from the tubing and the syringe prior to use.
What is the intended duration of use of the Aerogen Continuous Nebulisation Tube Set (CNTS)?
The Aerogen Continuous Nebulisation Tube Set (CNTS) has been qualified for use for a maximum of 7 days with the same type of medication.¹ If the medication to be delivered to a patient changes the Aerogen CNTS should also be changed. The accompanying syringe is a disposable item that has been qualified for a single use. Note: Aerogen Solo, when used continuously, also has a life of 7 days.¹ The user should note that use of the Aerogen CNTS and Aerogen Solo in continuous mode in excess of these periods is not qualified by Aerogen.
1. Aerogen Data on File
2. Aerogen Instruction Manual
GL1221A09-22












